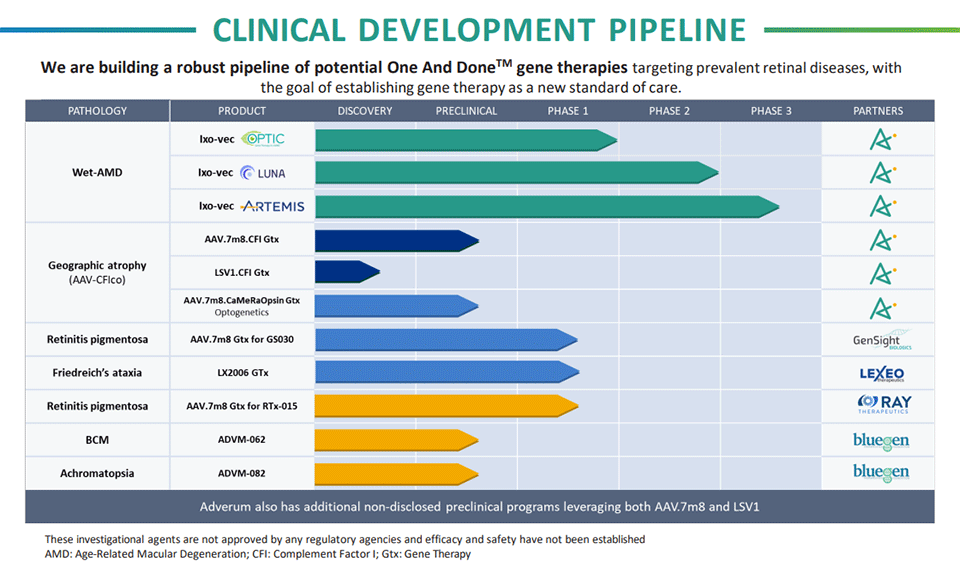
Pipeline
Pursuing Paradigm-Changing Gene Therapies
Advancing Ocular Gene Therapy
Ixoberogene soroparvovec (Ixo-vec, formerly referred to as ADVM-022) is Adverum’s clinical-stage gene therapy product candidate being developed for the treatment of wet AMD. Ixo-vec utilizes a propriety vector capsid, AAV.7m8, carrying an aflibercept coding sequence under the control of a proprietary expression cassette. The current standard of care in wet AMD, a highly prevalent disease, requires frequent anti-vascular endothelial growth factor (anti-VEGF) injections in the eye and is a lifelong burden for many patients and their caregivers. We believe ADVM-022 has the potential to provide a durable, safe and cost-effective in-office treatment option that addresses the needs of these patients, and their families, as well as retina specialists and health systems worldwide. Three-year aflibercept protein expression demonstrates continuous and consistent therapeutic levels from Ixo-vec in OPTIC study subjects with wet age-related macular degeneration (wet AMD).
OPTIC Clinical Program for Wet AMD
The typical way to evaluate anti-VEGF agents in clinical studies is to measure their short-term efficacy (i.e., 12 months or less). This approach, however, ignores or minimizes key factors that determine how well the therapy will work in the real world: a patient’s ability to maintain treatment regimens that require intravitreal injections every 4–12 weeks. Patients in OPTIC previously required very frequent anti-VEGF injections, with a median of 10 injections in the year preceding their enrollment.
Adverum’s OPTIC clinical program for wet AMD is investigating the safety and efficacy of Ixo-vec for the first two years post-treatment (the OPTIC trial) as well as evaluating the long-term patient outcomes for up to five years (the OPTIC Extension trial).
- Robust aflibercept protein expression sustained through three years
- Annualized anti-VEGF injection rate was reduced by 81% in all participants receiving 2E11 vg/eye and 94% in participants with NAbs titers <1:125, suggesting that those with baseline NAbs to AAV.7m8 <1:125 were likely to demonstrate more robust aflibercept protein expression and require even fewer supplemental anti-VEGF injections
- 67% (10/15) of 2E11 vg/eye subjects had baseline NAbs titers of <1:125
- Both doses of ADVM-022 were well tolerated with the 2 X 10^11 vg/eye dose requiring less topical corticosteroid therapy to alleviate inflammation. At most recent follow-up, no participants in the 2E11 vg/eye required any topical corticosteroids to treat inflammation
- No correlation between baseline NAbs and safety events was observed. Additionally, baseline NAbs were not associated with occurrence or duration of inflammation
- Vision (mean BCVA) was maintained, and retinal anatomy (mean CST) was maintained to improved versus baseline
- ADVM-022 had a promising safety profile; all ADVM-022-related ocular adverse events (AE) were either mild (81%) or moderate (19%), and patients have reached a follow-up period of between 1- and 2-years post treatment
Publications
Learn more about our technology, pre-clinical research, and clinical data in patients.